What is distillation?

Distillation is a commonly used method for mixture separation based on the differences required to change the phase of each component of the mixture and separate liquids. The liquid mixture can be heated to force the elements of different boiling points into the gas phase. This is where the fluids are condensed back into liquid and later collected.
There are two kinds of distillations, simple and fractional distillation. In this guide, we will focus mainly on the complex type, fractional distillation.
Uses of distillation
Distillation is applied in numerous commercial processes. These include gasoline production, production of distilled water, alcohol, kerosene, paraffin just to mention a few. Also, gas could be liquefied and separated. Such gases include oxygen and nitrogen.
Distillation is by far the most important method of separation in petroleum and most chemical industries. It helps in the separation of different components in a mixture whose volatility and boiling points are different. This is also referred to as functional distillation fractionation.
In fact, distillation has been termed as the most economical separation method for liquid mixtures. However, the process can be energy intensive and requires quite a good level of energy for function. The results are guaranteed though, and that is why it is worth investing in. Other alternatives require a high investment cost, and in the end, distillation remains to be the most favorable choice, especially in large-scale operations.
Distillation column mode of operation
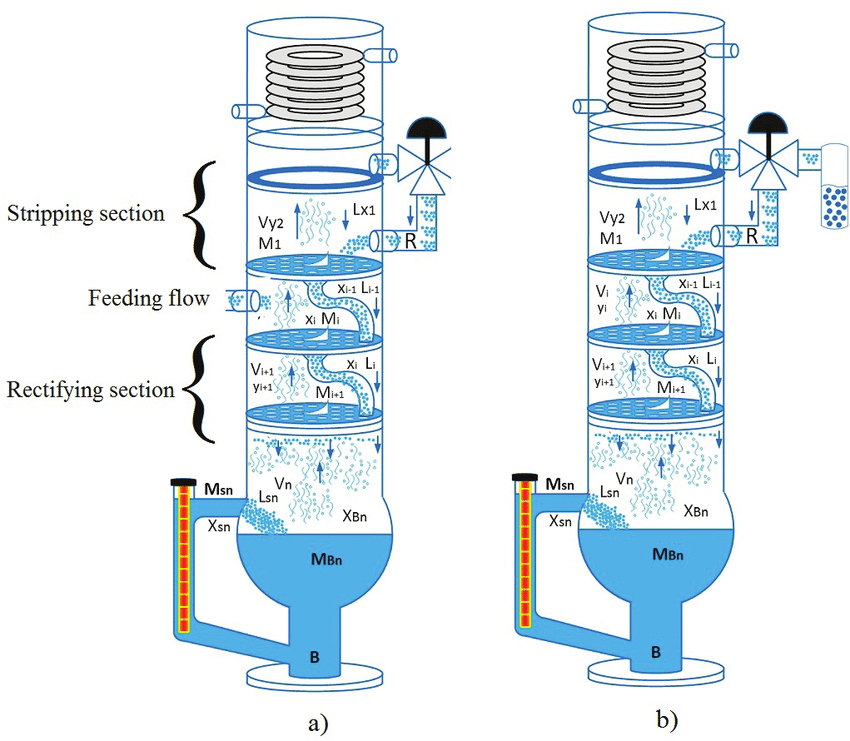
As discussed earlier, the distillation column working principle involves the separation of mixture components with varying boiling point. This way, vapor from a boiling mixture will mainly comprise of the element with a lower boiling point. Once this vapor is cooled, it condenses and the liquid formed will contain more of the volatile component; thus the original mixture will be left with more of the less volatile component.
What is distillation column? It is a pressure vessel where the mixture is separated. It is used to make the whole separation process possible based on the components' volatility differences. A standard distillation apparatus is made up of three main parts which are, a pall ring for vapor to pass, a condenser where the vapor is cooled, returning it to a liquid form and a source of heat which is used to vaporize the original mixture.
Ideally, the mixture element with the lowest boiling point will vaporize first and the temperature remains constant until all substances are entirely evaporated.
There are two types of distillation columns, and each model is designed to offer a different kind of separations. Every design has a different level of complexity. The main types are batch columns and continuous columns.
Batch columns – in this operation, there is the introduction of a batch-wise feed to the column. This means that the column will be charged with a batch as the distillation process takes place. Once the desired results are achieved, the next batch is introduced, and the, cycle continues.
Continuous column – in this case, the distillation process is carried out in a continuous feed. This means that once the process is started, there are no interruptions until all the mixture is separated unless a problem occurs with the columns or other surrounding units. The continuous columns are designed to handle even high throughputs and happen to be the most commonly used in commercial industries.
The continuous columns are further categorized based on;
• The nature of the feed that needs to be processed
In this case, there is the multi-component column which separates feed that contains over twp components and the binary column which is meant for separating feed with only two elements.
• The number of contained streams
These are based on the number function streams where the products are meant to pass. There is the multiproduct column which comprises of more than two streams.
• The fee exit during separation
This category is based on the side in which the feed goes out after separation. There is the extractive distillation whereby the extra feed settles on the bottom product stream and the azeotropic distillation where the extra feed settles on the top product stream.
Now that you already understand about the distillation columns let's have a look at its components. The first things to note are the column internals, which is categorized into two as follows;
Tray column – this is a situation where the trays of different designs are crafted to hold up the liquid to offer better contact between the liquid and vapor, thus ensuring a better separation. There are different types of trays in distillation column and they include dual-flow tray, sieve tray, bubble cup, and valve tray.
Packed column – this is where the packings are used in place of trays to enhance contact between liquid and vapor. They include structured packing, tower packing and random packing.
What are the main components of the distillation columns?
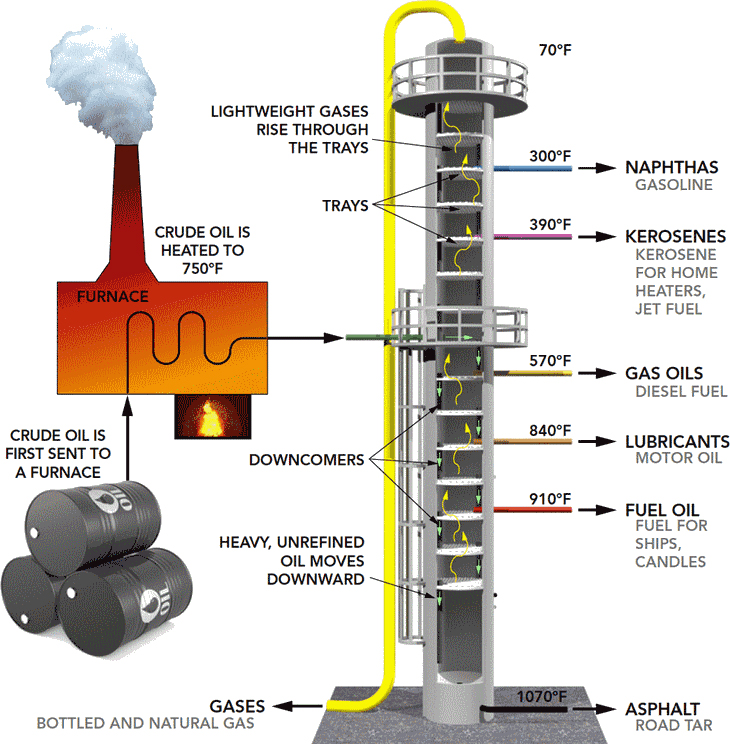
Distillation columns comprise of many components, all which are used for either transferring heat energy or help in the material transfer. These components include;
A vertical shell – this is where the separation of liquids happens
Column internals – these include trays or packing. There are the random packing and tower packing whose purpose is to help in component separations.
Reboiler – this is designed to offer the needed vaporization during the distillation process
Condenser – this is where the vapor is cooled and condensed back to liquid.
Reflux drum – this part holds the condensed vapor from the top of the column ensuring that the liquid can be recycled back to the column
What happens if we increase pressure in a distillation column? The process itself involves pressure, but then there is a recommendable amount based on what needs to be separated. Increasing pressure will lead to various things;
First, increasing pressure leads to an increase in the bubble point of the mixture and we will need more reboiler function. If our hot utility is not compatible with the change, it will need to be replaced
The second effect of increased pressure is increase in the dew point of the mixture. The results are the need for more condensing, and if the cold utility is not compatible with the change, it needs to be replaced
Increased pressure will result in a decrease in the relative volatility since pressure and boiling point have an inverse relationship. In this case, ore stages will be required for the same purity of product. The height of the distillation column will also increase.
Increase in pressure will lead to a need for increased reflux ratio
Fractionating column in fractional distillation; what is the use?
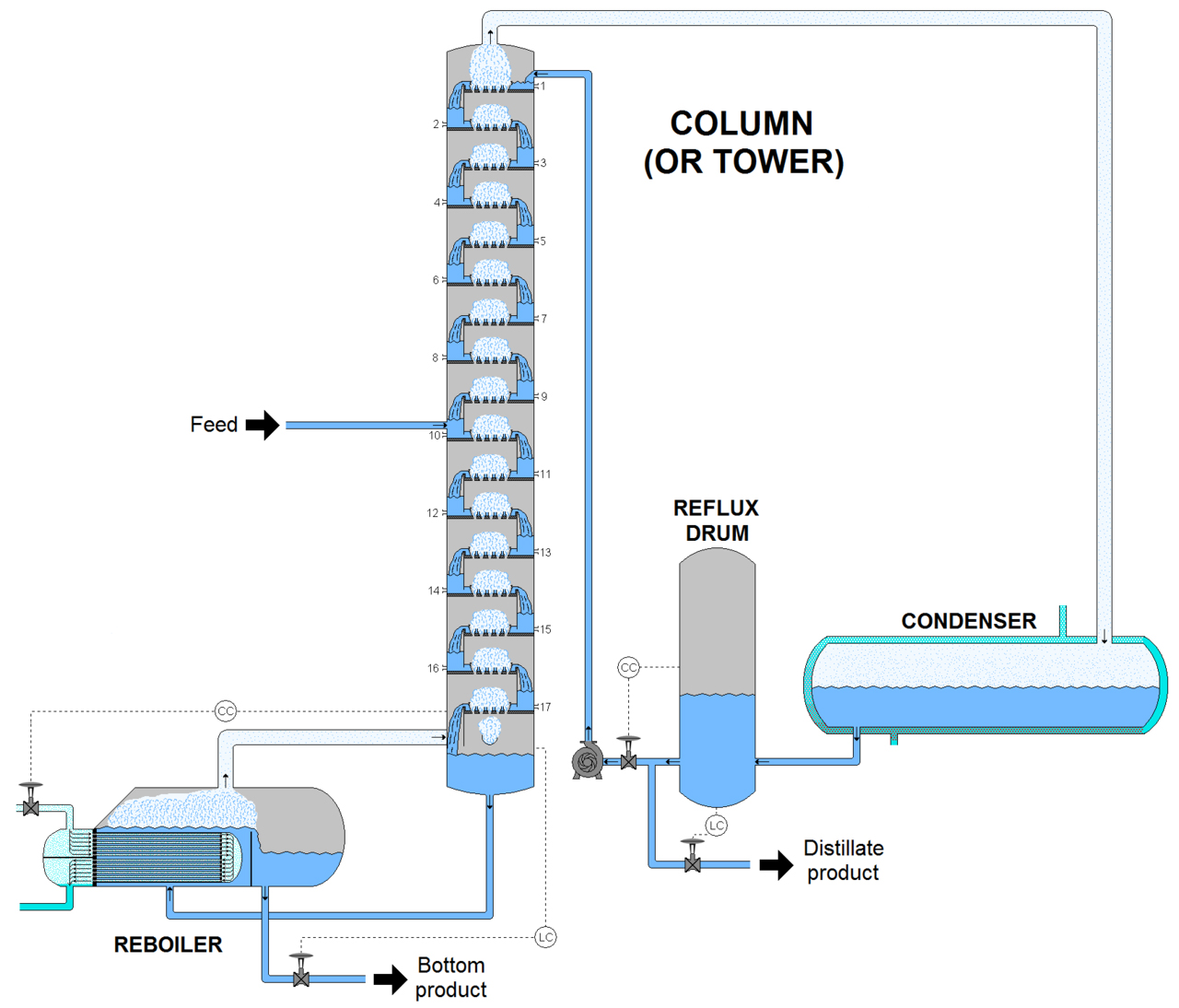
Distillation is all about the separation of miscible mixtures through vaporization. I'm sure I have emphasized that enough and in case you are wondering what could be the purpose of the fractionating column through the process, it is used to separate liquids based on the order of their vaporization to separate them as they are vaporized. This process is known as fractional distillation, and it is quite a special kind in which two miscible components with different but close boiling points are separated.
The column contains long glass tubes or raschig ring and a wide bore which is either packed with small stones, glass beads or rings. The aim of the fractionating column is mainly to increase the cooling surface area and offer hurdle to the descending liquid and ascending vapors.
However, you may notice some issues during distillation the common ones being flooding and foaming.
What is flooding in distillation column?
During distillation, vapor goes up while liquid flows down, but if any of these flows is interfered with, the results would be flooding in a distillation column. Flooding is a situation where the liquid is carried away by gas. This issue occurs at very low liquid flow rates such that the fluid backs up in the vapor side to a drop in the tray pressure.
What causes foaming in the distillation column?
During distillation, you may notice some foaming which can be a bit scary especially if you do not know its causes and effects. First of all, what does foaming in distillation means? This is the expansion of liquid which offers high constant between liquid and vapor. Although it is not common, the issue cannot be overlooked. Its major cause is the malfunction of the distillation equipment and could increase until the liquid in one tray mixes with the liquid on the tray above during the entrainment process. This is a process which decreases vapor-liquid equilibrium thus slowing down the process. Foam can be caused by many aspects some of which include;
• Trays – the design, placement, and condition of the trays can have a huge impact on the whole process and could result to foaming if not appropriately done take an example of a situation where the overhead disengaging area is not placed high enough. This can only mean that the trays will be closely placed above each other and therefore, in a case of liquid expansion, the liquid in the two trays, bottom and top will foam.
• Vapor conditions – foaming could result from passage of gas or vapor. This could happen due to a very high rate of gas velocity or a high rate of evaporation.
• Liquid drop – this happens if liquid from the feeder hose falls on the sump and the higher the liquid drop, the lesser the created foam. This issue is more common in tray column sumps than in pack columns.
• Malfunctions - Mechanical malfunctions are the most common cause of distillation foam. The issues include failure of the mechanical foam breaker to rotate or if the baffle foam breaker is poor designed or damaged.
• The liquid physical properties – the liquid's physical property can contribute to the creation of foam. The zero points of change far from PH will mean that the liquid has a bad acidity level. There are naturally occurring surfactants and ring polymers, both which reduce the fluid's surface tension. Any corrosion particles and any other solid articles could result in unwanted foam.
• Wrong anti-foam agents – use of ineffective antifoam agents is also a common cause of foam. The problem could be as a result of the wrong amount of the compound or using the wrong kind that does not go well with the fluid that intends to be distilled.
Conclusion
I believe that distillation column introduction guide is enough to answer any questions that you may have regarding distillation, distillation columns, types of trays and the functions of each. If you wish to start a business that involves the process you are good to go. With this, you can comfortably operate the equipment and handle any issue that you may face on the way, the common one being foaming. You can contact us for further assistance regarding distillation columns working principle, and we will be glad to help. Below I leave you with distillation column diagrams for additional guidance.








