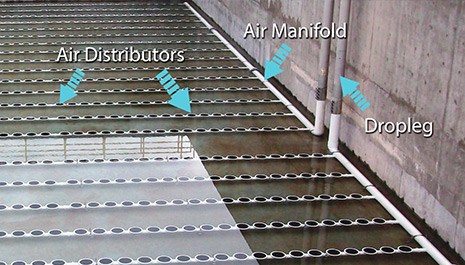
Aeration of waste water
Biodegradation is very imperative when it comes to breaking down organic compounds to form water and Carbon (IV) Oxide. For this reaction to occur, oxygen is necessary.
When Oxygen is not sufficient, the process is too slow and may result to incomplete conversion of the compounds and low pH conditions.
Consequently, odors are produced from the tank and the water becomes too challenging to treat.
This in return brings the dire need of pumping air (Oxygen) into the tank. This is what is referred to as aeration. So you need some fine bubble diffusers in your aeration tank to treat water.
In this case, we recommend sufficient, uniformly distributed supply for the whole process to be in effect, financially feasible and yielding.
7 Myths About Aeration Tank
1. The higher the pressure, the higher the absorption
This is absolutely false. When it comes to absorption of oxygen, increase in pressure of the gas does not mean increase in absorption whatsoever.
What happens when pressure is increased?
To answer this, we take you through some simple straight forward calculations.
For efficient absorption to take place, the contact time between the air bubble and water must be around 100 seconds (1 min 40s).
Taking the speed of a rising bubble to be 1ft/s, it therefore implies that for effective absorption, the tank must have a depth of up to 100 ft.
Where the tank is not that deep, other techniques must for that reason be applied to make certain that the contact time is upheld. This implies that the contact time and not the pressure is all that matters.
From the foregoing discussion, it is clear that increasing pressure does not directly make absorption more efficient.
2. At 100% saturation, spontaneous effervescence is impulsive.
Most people believe that effervescence will undoubtedly occur once saturation is reached. This is not exclusively undeniable, it is one of the many conditions that must be met for it to occur. It is necessary but not efficient.
So, when will spontaneous effervescence occur?
As mentioned previously, there are conditions that must be met for it to occur. They include:
• A defined minimum turbulence level. There is a minimum threshold turbulence level without which spontaneous effervescence will not occur.
Take for instance,300 ml Coke contains 1.5l dissolved Carbon (IV) Oxide with 500% saturation.
When it is shaken vigorously before opening, the surface area of the gas increases. When opened, the dissolved gas escapes forcefully.
However, when left to settle before opening, all the bubbles rise up to the surface which on opening it, only small amount of the gas escapes. This implies that DO (gas) will take time to come out if it is undisturbed.
Consequently, when water with DO concentration being around 100ml/l is mixed gently, no effervescence is observed.
• Concentration of the gas. Foe spontaneous effervescence to occur, minimum DO concentration must be achieved without which it will not take place.
• The sites of formation of cells in the water.
From the preceding argument, it is manifest that saturation alone will not cause spontaneous effervescence.
3. The bugs need air 24/7
This may sound true from far but realistically, it has been greatly invalidated by science. Main facts invalidating it include but not limited to;
I. Oxygen does not deplete immediately when the system is shut. The rate at which it deplete is as well dependent on many factors that include
• Temperature
• Organic loading (quantity of waterless organic matter inflowing the anaerobic digester per unit time)
• Diffuser type
• What time of the day it is
This implies that it wrong to put the time of depletion of Oxygen fixed as it is wholly dependent on other changeable factors.
II. If the above statement was true, this would imply that oxygen in the aeration tank exists in one form only, DO, which is not true. Oxygen exists in many forms which include
• Nitrate ions (NO3)
• Nitrite ions (NO2)
• Sulphate ions (SO4)
From the examples above, it is evident that oxygen (O2) is present although it is chemically bound.
When the supply of oxygen is shut, oxygen in DO form starts approaching zero with immediate effect. However, there is much more oxygen in the system though bound.
The oxygen on the chemical compounds therefore provides for Biochemical Oxygen Demand (BOD)
4. MLSS does not suspend again if you shut the aeration system down
Due to familiarity with so many ineffective aeration tanks, so many myths rotate around it. However with the ever advancing technology which has resulted to appropriate aeration tank design, we are shedding light to these contradicting issues with expertise.
Mixed liquor suspended solids (MLSS) were believed to sink permanently whenever the system is shut down. In the current digital times, systems have been observed to re-suspend the solids in as much as the oxygen supply has been stopped. This can go for as long as 3600s.
5. More Oxygen concentration results to a more efficient system
Just like most of the chemical reactions, increasing one of the reagents is too much does not lead to a more reaction. This case applies here too. In fact, too much oxygen causes operational problems such as failure in he sedimentation tank.
What happens if you increase oxygen concentration?
i. When food for microorganisms gets depleted, they excrete a sticky substance around their cells allowing the mixed liquor to settle well.
Aeration allows the bugs to float and remain in due course stuck together. This sticky substance is called floc. When it reaches secondary clarifier, it is heavier than water.
Anything denser than water sinks, so do the floc. Putting too much oxygen will cause the floc to disperse which needless to say will not settle well.
ii. Anoxic zones are areas in which removal of nitrogen from the wastes takes place- denitrification. This vital process strictly takes place in anaerobic conditions. However, high DO causes the zones to turn aerobic in part.
Denitrification may as well stop since bugs use free O2(DO) instead of chemically bound oxygen.
6. It is possible to swim in an aeration tank?
People who have not heard about the myth wonder if they can swim in an aeration tank.
As a matter of fact, it is practically impossible. In fact, the question should be what are the chances of rescue if one accidentally fell in an aeration tank.
When swimming, buoyancy of the liquid is imperative. This is the upward force acting on an object that is partially or fully immersed in a fluid. Since it opposes your weight (mg) when swimming, it allows you to float.
However, in the aeration tank, the liquid has many air bubbles by aeration diffusers. For this reason, the buoyancy of the liquid is lost.
Consequently, the moment you get into the water either in an attempt to swim or even accidentally, you will unquestionably sink to the bottom no matter how much you thrash. This explains why the chances of rescue are slim as compared to when one falls into non aerated water.
7. It is not important to keep checking the level of DO
Even before we address this issue, we address the frequently asked question of whether it is even possible for you to monitor the level.
Actually, there are so many things that need to be monitored in an aeration tank though; the DO and pH are the most imperative conditions.
First, let us see why monitoring is important.
As earlier mentioned, when the pH is low, odors are likely to be produced. It also makes it very difficult to treat the water. This implies that for optimum functioning of the tank, the pH must be maintained at a defined range, in this case between 6.6 and 8.5.
Like any other chemical reaction, too high or too low concentration of DO will negatively affect the efficiency of the system. This brings the dire need of monitoring DO level.
Did you know that you can monitor the pH and the dissolved oxygen level without strain? This is done using a very efficient, meter that is specifically designed for this. It is ultra portable which is actually a plus.
Conclusion
Information is power. This is why we are indeed committed to providing information about aeration tanks to ensure that you do not rely on groundless facts, myths and misconception when addressing this sensitive topic.
Fueled by passion and backed up by expertise, we give precise information, clarifying the contradicting information and gets to the bottom line of the same.
We equip you, to ensure that your dreams of making an effective aeration tank come true.